Electroplating is a process that uses an electric current to reduce dissolved metal cations so that they form a thin coherent metal coating on an electrode.
All kinds of metals can be plated in this way, including gold, silver, tin, zinc, copper, cadmium, chromium, nickel, platinum, and lead. Electroplating is carried out in an electroplating bath of an aqueous solution, it belongs to the chemical reaction coating coloring, and this color makes it look more floating and less realistic.
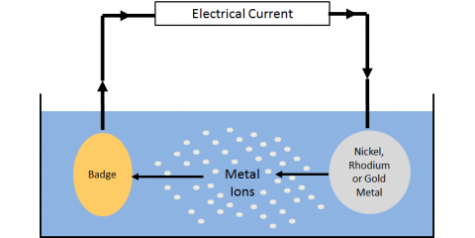
1. The plating metal is attached to the anode or a soluble salt of the metal to be electroplated is added to the bath.
2. The object to be plated is attached to the cathode.
3. The anode and cathode are connected by an electrolyte solution composed of electroplated metal cations.
4.After passing through the DC power source, the metal of the anode releases electrons, and the positive ions in the solution are reduced (electrons obtained) at the cathode to form atoms and accumulate on the surface of the cathode.

The aesthetics of the plated object after electroplating is related to the current density. In the range of operable current density, the smaller the current density, the more beautiful the object to be plated; otherwise, may lead to the uneven shape.
Current density refers to the current distribution over a certain area. Commonly used current density units are amps per square decimeter (ie, square inches) [ASD] and amps per square foot [ASF].
In general, the plating bath is acidic and can corrode and dissolve the cathode plating metal. When the current density is too small (less than 5 ASF), the plating metal will exhibit a loose and dull appearance due to the dissolution of the acidic bath.
For more information, Please visit China Jewelry Manufacturer.